Anodizing is a process that grows an oxide layer on metal. This layer protects the metal. Stainless steel has chromium that makes its oxide layer. This made it hard to anodize more oxide on stainless steel before. But now new methods let people anodize stainless steel too. It makes the steel stronger and more colorful.
Stainless steel is used for many things. But it has been too hard to anodize in the past. Anodizing helps metal resist damage. Can you anodize stainless steel after all? Scientists found special ways to make it work. Now stainless steel can have colorful anodizing too.
New anodizing methods work on stainless steel. They make a controlled oxide layer on the surface. This layer stops rust and scratches. It also allows creative colored designs. Products can be improved by anodized stainless steel.
Overview of Popular Anodizing Processes
Anodizing is a procedure that grows an oxide layer on metals like aluminum and titanium to enhance corrosion resistance, wear resistance, and different homes. The anodic layer allows colored finishes on aluminum. proper anodizing of chrome steel may be very tough.
Stainless steel does now not corrode effortlessly, so it no longer forms an oxide layer via regular anodizing. At best, the stainless steel surface gets etched. There are alternative processes to try to create color effects similar to anodizing on stainless steel, like flame coloring/heat treating.
These have mixed results, and most companies consider their coloring processes proprietary. While you can electroplate stainless steel with a pure aluminum layer first, then anodize that layer to get effects similar to directly anodizing aluminum, it is rarely done. The process would not be as durable as direct aluminum anodizing.
In summary, true anodizing of stainless steel without first electroplating aluminum is very limited. The material properties of stainless steel prevent growing an oxide layer to allow functional anodized coatings. Other coloring methods only approximate the effects of anodizing. Do you know what toxic substance is released when welding stainless steel?
Examining Stainless Steel and Its Passive Layer Barrier
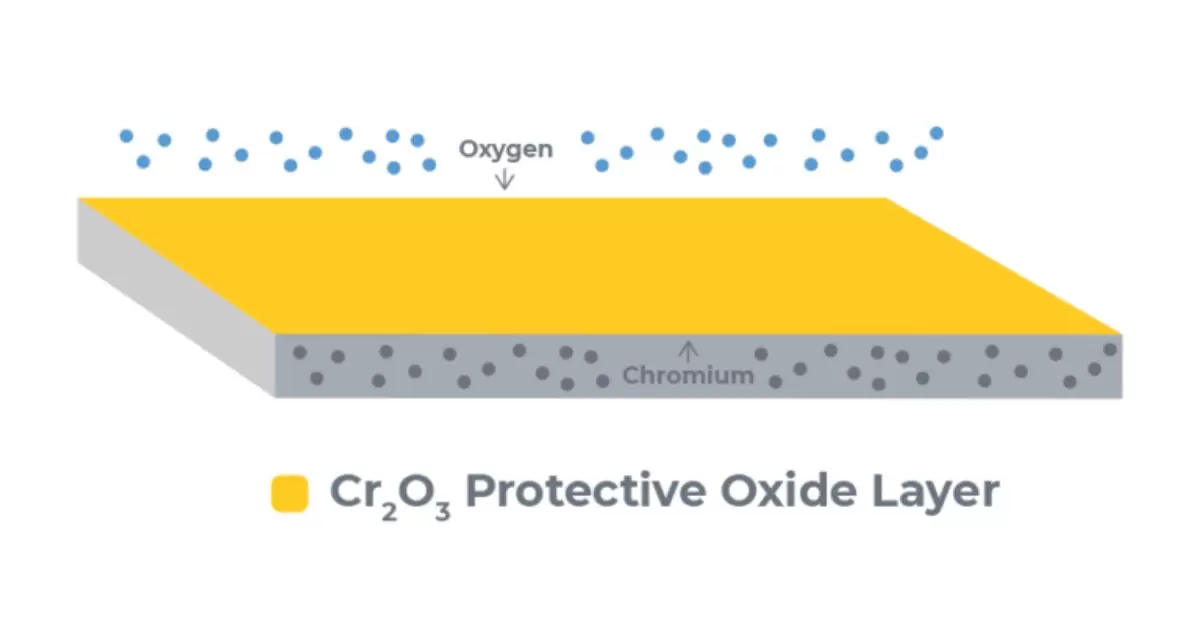
Stainless steel is valued for its excessive strength, hardness, corrosion resistance, ease of sterilization, and high-temperature functionality. The various chrome steel grades all include iron, at least 10.5% chromium, and frequently nickel, manganese, and molybdenum. The chromium in Stainless steel bureaucracy is a “passive layer” – a defensive chromium-oxide barrier that provides first-rate corrosion resistance. This passive layer is certainly self-maintenance when broken.
The passive layer is only a few molecules thick however prevents oxygen and moisture from reaching the iron within the stainless steel. If oxygen reached the iron, it would oxidize and purpose rust, weakening the steel. Passivation treats stainless steel to regenerate and decorate this shielding passive oxide layer barrier. Passivation removes unfastened iron and infection from the surface after machining or welding.
The ensuing passive layer resists corrosion and rust, extending the stainless steel‘s existence. Passivated Stainless steel meets industry standards like ASTM A967 that validate the passivity and corrosion resistance.
Overview of Anodizing and Its Benefits
Anodizing is an electrolytic system that grows an anodic oxide layer on metals like aluminum and titanium. This anodic layer gives colored finishes, corrosion protection, put-on resistance, electrical insulation, and higher adhesion for coatings. The anodic oxide from aluminum and titanium anodizing creates a tough, long-lasting floor that substantially improves corrosion and abrasion resistance.
Anodizing additionally allows colored finishes without using dyes or pigments. Anodizing serves as an alternative to metal plating or coatings in many packages. It’s far a price–effective way to beautify metallic surfaces with tailored properties for exceptional features. The anodic layer properties may be adjusted by controlling the anodizing method parameters.
Key benefits of anodizing aluminum and titanium include severe floor hardness, top-notch corrosion/wear improvements, the capability to create stunning colored finishes, electric insulation, and higher adhesion for subsequent coatings or glues.
Understanding Stainless Steel Grades and Properties
Stainless steel is an alloy containing a minimum of 10.five% chromium for corrosion resistance, at the side of iron and different factors like nickel, manganese, and molybdenum. There are numerous grades utilized in numerous applications. Famous austenitic grades like 304 (18/8) and 316/316L have splendid corrosion resistance and ductility, making them great for kitchenware and domestic devices.
Duplex steels mix austenitic and ferritic microstructures for enhanced houses. Martensitic and precipitation hardening grades use heat treatment to increase strength. Key properties making stainlessly invaluable across industries include high strength, hardness and corrosion resistance, easy sterilization and cleaning, good formability and ductility, and capability for high/low-temperature operation.
In particular, stainless steels get exceptional corrosion resistance from a protective “passive layer” – a chromium-oxide layer that forms naturally when the chromium in the steel interacts with air. This layer poses challenges to the traditional anodizing of stainless steel.
Difficulties of Anodizing Stainless Steel
Stainless steel‘s pre-present passive layer interferes with developing an anodic oxide coating, which is fundamental for anodizing. This chromium-oxide barrier restricts achieving sufficient oxide growth. Typical anodizing electrolytes used for aluminum do not work well on stainless steel. The various alloying elements like nickel and molybdenum also complicate the anodizing reaction.
So conventional anodizing of stainless steel has faced substantial barriers. Anodizing stainless has long been considered unfeasible for decorative finishes. Recent innovations in anodizing processes and techniques show promise for wider adoption. Advancements are changing past perceptions that stainless steel cannot be functionally anodized.
Cutting-Edge Processes Enable Anodized Stainless Steel
Latest research has proven that anodizing stainless steel is feasible by way of carefully selecting the electrolyte, temperature, voltage, and surface pre-remedy. This allows for in development of nanostructured anodic layers on stainless steel. The resulting anodic surface layers have enhanced corrosion resistance, improved wear/abrasion resistance, better adhesion for coatings/glues, and biocompatible medical surfaces.
Researchers have successfully produced colorful anodized finishes on stainless steel using sulfuric acid electrolytes. This demonstrates the decorative potential. Thus, modern anodizing techniques are overcoming past barriers that prevent the functional anodizing of stainless steel. Anodic stainless steel surfaces with tailored properties can now be achieved.
Emerging Uses for Anodized Stainless Steel
Anodized stainless steel enables new medical, marine, aerospace, architectural, and food processing applications. The anodic layer provides enhanced biocompatibility, corrosion protection, wear resistance, cleanability, and colored finishes. For example, anodized stainless steel fuel cells and battery plates improve corrosion resistance and electrical outputs.
The stable anodic layer also allows colorful and durable public art installations. Additional uses include saltwater marine hardware with better durability from the anodized layer. Aerospace and architectural components benefit from enhanced wear resistance and adhesion for protective coatings applied over the anodized layer. Food processing equipment requires fewer coatings and has improved cleanability thanks to anodized stainless steel surfaces. The inert and nonporous anodic layer prevents bacterial adhesion.
| Barrier | Description |
| Pre-existing passive layer | Interferes with oxide growth reactions |
| Incompatible electrolytes | Problematic with typical chromic and sulfuric acids |
| Alloying elements | Complicates achieving controlled oxidation |
| Limited oxide layer thickness | Passive barrier hinders thick anodic layers |
Recent Advances Enable Anodized Stainless Steel
Recent research has achieved anodized stainless steel using optimized sulfuric acid parameters and surface pre-treatments. This grows anodic surface layers with enhanced corrosion resistance, bioactivity, adhesion, and colored finishes. Controlling factors like temperature, voltage, and duration enables sufficient oxide growth on stainless steel’s passive layer.
Pre-treatments maximize this oxide layer formation through anodization. Resulting properties include better corrosion protection, improved biocompatibility for medical uses, superior adhesion for protective coatings, and attractive integrated color effects without dyes. Some new techniques can directly color stainless steel through the anodization itself. This expands the creative potential for stunning and durable anodized stainless finishes.
Emerging Applications for Anodized Stainless Steel
Anodized stainless steel enables new medical devices like implants with improved biocompatibility, better bone growth, and enhanced antibacterial properties from the anodic nanostructures. Architectural applications benefit from anodized stainless steel’s colored finishes without coatings, excellent abrasion resistance, and reduced maintenance needs compared to protective films.
In aerospace, anodized stainless steel provides superior adhesion for protective films, enhanced corrosion resistance, and thermal barrier properties from the anodic layer. As anodizing techniques are optimized and standardized for commercialization, additional cutting-edge applications across sectors will emerge. The tailored properties of anodized stainless steel create new capabilities.
The Stainless Steel Anodizing Process
Stainless steel anodizing utilizes a sulfuric acid bath along with an electrical current to grow and densify the passive oxide layer. Thorough cleaning and optionally grit blasting prepare the surface. During anodizing, the stainless steel is immersed in the room temperature sulfuric electrolyte with 20-100V applied for 15-90 minutes. Agitation helps improve oxide formation.
Optional coloring baths before sealing can create black, bronze, gold, and other shades on the anodized layer through electroplating or chemical processes. A hot water bath or steam exposure seals the pores of the anodic layer, which improves corrosion protection. Tuning parameters like temperature and voltage tailor properties.
Benefits of Anodizing Stainless Steel
Anodizing stainless steel complements corrosion resistance, enhancing an already pretty corrosion-resistant material to be used in excessive environments. Anodization additionally lets in aesthetic customization with metal coloration finishes. Different anodized color finishes on stainless steel can denote specific grades or designations through color coding.
The anodic layer also better prepares the stainless surface for improved adhesion of glues and epoxies. In addition, the anodization provides increased surface hardness and wear/abrasion resistance. Performance tends to be best on higher alloy stainless steels like 316 and 316L, which contain molybdenum.
Limitations of Stainless Steel Anodizing
The color options for anodized stainless steel are more limited than metals like aluminum or titanium, typically just black, bronze, gold, and grey shades. Since stainless steel already has excellent corrosion resistance from its passive layer, the improvements in corrosion protection from anodizing are incremental for most applications.
Anodized stainless steel is a more expensive surface treatment option but provides a minimal performance advantage over stock stainless steel in many situations. Also, the anodized color finishes may slowly fade over time without properly sealing the anodic layer after anodization.
Example Applications
Anodized stainless steel is used decoratively in architecture like bridges and building exteriors to maintain appearance after outdoor exposure. The black coloring resists fading. Artistic steel sculptures and public displays also utilize anodized stainless for integrated black finishes and incremental corrosion resistance.
Harsh marine settings like ship propellers and desalination plants benefit from the extra surface hardness and corrosion protection anodizing provides to stainless components. Medical implants and devices take advantage of anodized stainless steel’s biocompatibility and cleanability enhancements for improved patient outcomes.
FAQs:
Does anodizing work on stainless steel?
Yes, recent research has developed processes to anodize stainless steel, though it faces challenges due to stainless steel’s pre-existing passive layer.
What metals Cannot be anodized?
Iron-based metals like steel cannot be anodized because they do not form the protective oxide layers required for the anodization process.
Can you change the color of stainless steel?
Yes, anodized stainless steel can be colored through electrolytes or by dying the anodic oxide layer grown on the surface.
What is the voltage of anodizing stainless steel?
Research has used voltages ranging from 10-100 volts to anodize stainless steel, with parameters optimized based on the grade of steel and type of electrolyte.
Why steel Cannot be anodized?
Steel cannot be anodized because it does not form the protective oxide layer needed for the process when exposed to acidic electrolytes. Only valve/non-ferrous metals can.
Which metal is suitable for anodizing?
Aluminum is the most suitable metal for anodizing. Magnesium, titanium, zinc, niobium and tantalum can also be anodized but may require more controlled processes.
Conclusion:
In conclusion, recent advances now enable stainless steel to be anodized to grow surface oxide layers with protective and aesthetic benefits. Carefully controlled anodizing parameters allow nanostructured films to form on stainless steel without the limitations posed by the natural passive layer.
The key question is: can you anodize stainless steel? The answer is now definitively yes, with the use of optimized sulfuric acid electrolytes and pre-treatment procedures to overcome the barriers posed by stainless steel’s natural passive layer. The anodic layers created enhance stainless steel performance across applications from medical devices to aerospace components while opening up creative options for colored finishes.
As processes continue to be refined, anodized stainless steel will offer expanded capabilities not previously possible. So whether for improved function, striking designs, or both – stainless steel can now be anodized.